Looking Good Info About How To Build A Galvanic Cell

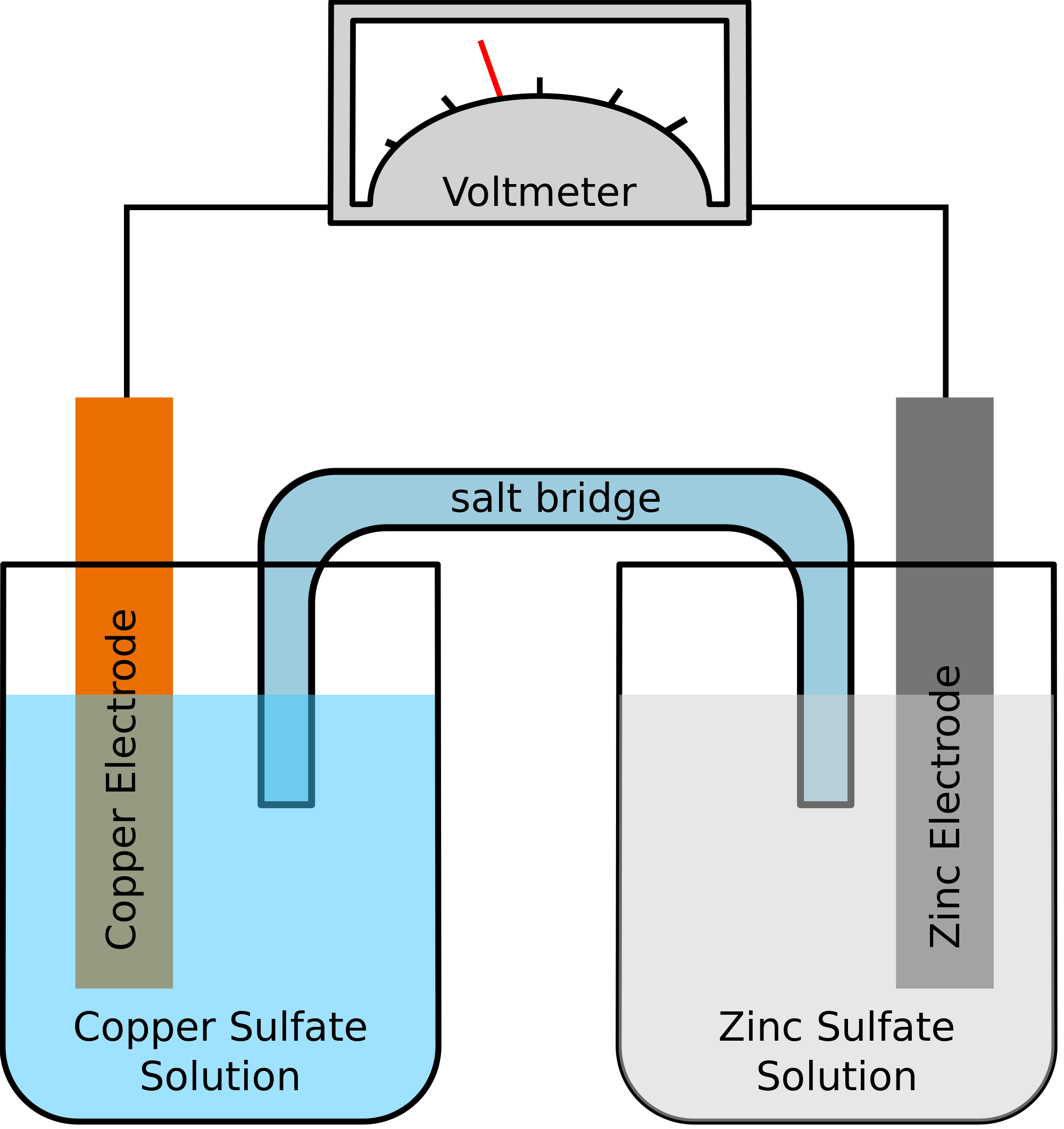
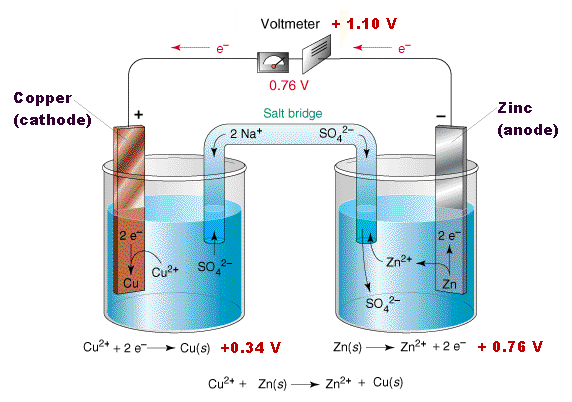
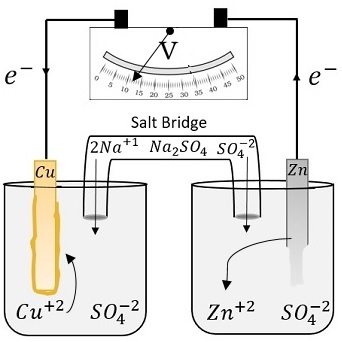
Fill one of the center wells with copper (ii) sulfate solution.
How to build a galvanic cell. For example, steel and copper. Construction of a homemade galvanic cell there are many ways to build a homemade galvanic cell. From several such elements it is possible to make a battery, which gives the necessary voltage for powering.
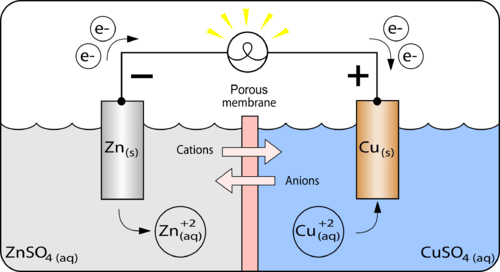
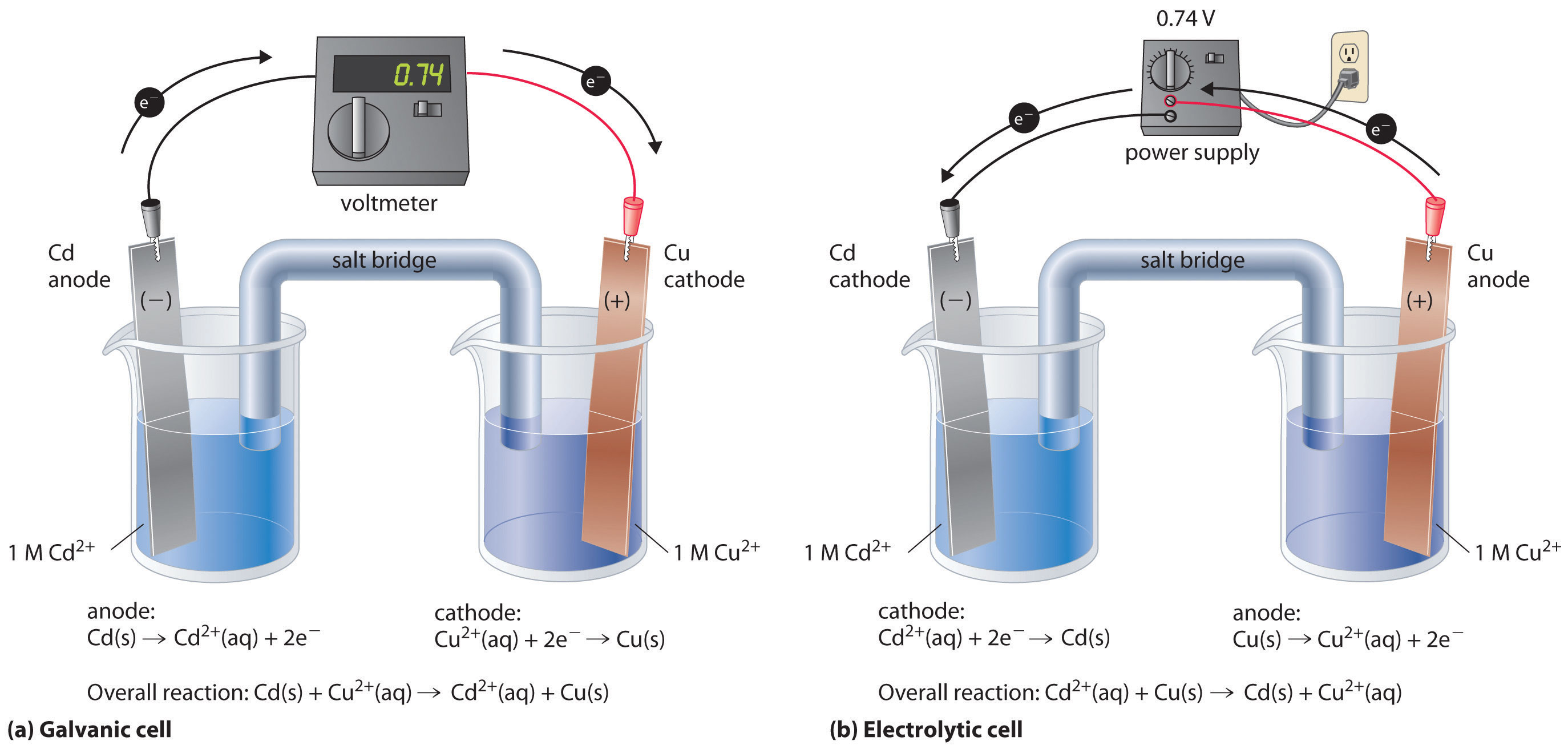
In this galvanic cell experiment, you’ll use different materials to create a battery, similar to the previous lemon battery. One can separate the copper and the silver solution and still carry out a redox reaction. A galvanic cell is constructed by combining an oxidation electrode with a suitable reduction electrode to convert chemical energy into electrical energy by a redox reaction.
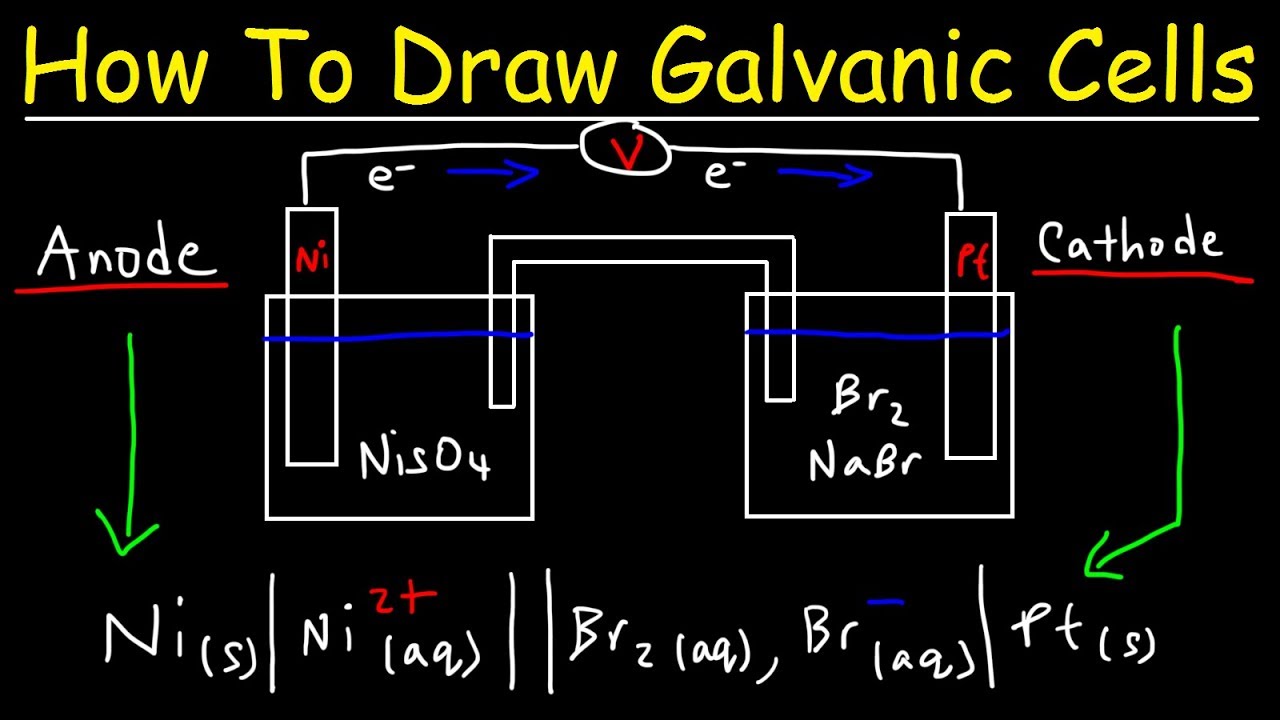
This allows us to take advantage of the movement of electrons from the copper to. A galvanic cell is constructed in which an ag+/ag half cell is connected to a ni2+/ni half cell. A galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical cell in which an electric current is generated from.
One of the simplest is using vinegar as a solution, steel nails, and copper wires. The video below walks you through everything. Up to 8% cash back order now.
Label the nine rows in the same way. Let’s start with an overview of the electrochemical cell driven by a spontaneous redox reaction. How to make a galvanic cell.
It explains how to identify the anode and th. Calculate delta g standard for this reaction at 25 degrees celsius: The galvanic cell may have an anode or cathode of dissimilar metals in an electrolyte or the same metal in dissimilar conditions in a common electrolyte.